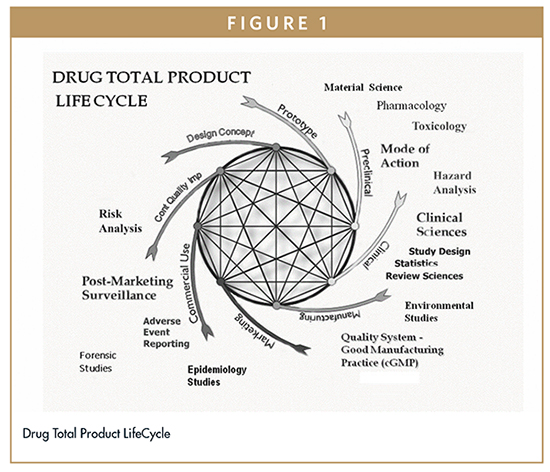
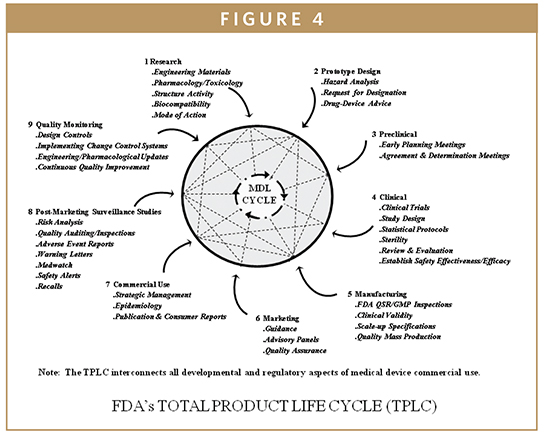
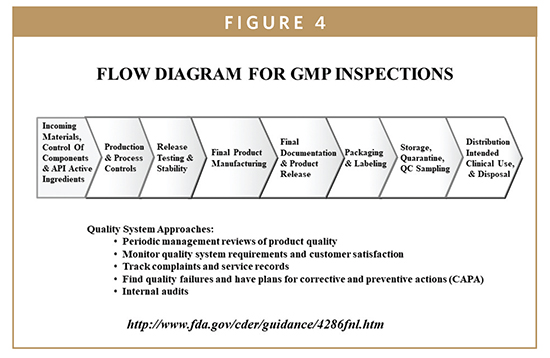
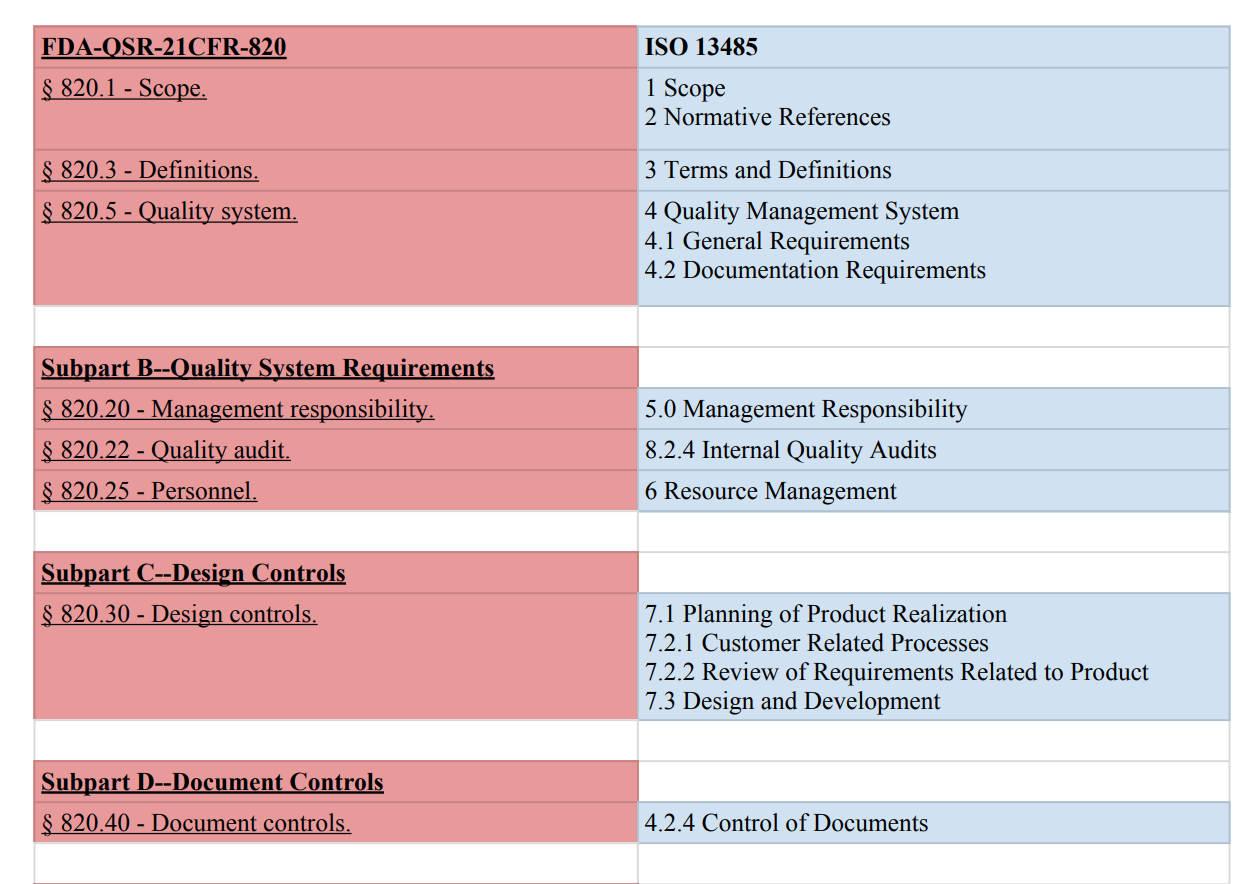
The quality system elements and management responsibilities described in this guidance are intended to encourage the use of science and risk based approaches at each.
Quality management system fda guidance.
In addition there should be written versioned procedural documents sops.
Used quality management systems including iso 9000 non u s.
Pharmaceutical quality management requirements and fda s own medical device quality system regulations.
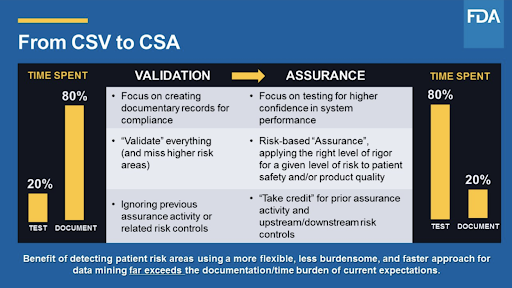
P the fda is preparing to release new guidance quot computer software assurance for manufacturing operations and quality systems software quot.
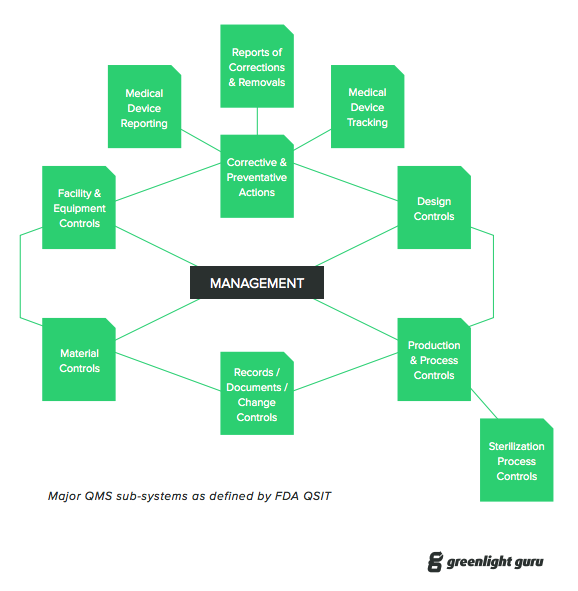
Each company should have a quality management system qms which includes a mission statement of the goals and scope of the program as well as defining applicable laws regulations and importantly best practices procedures where the law is unclear or silent.
A quality management system qms is defined as a formalized system that documents processes procedures and responsibilities for achieving quality policies and objectives.
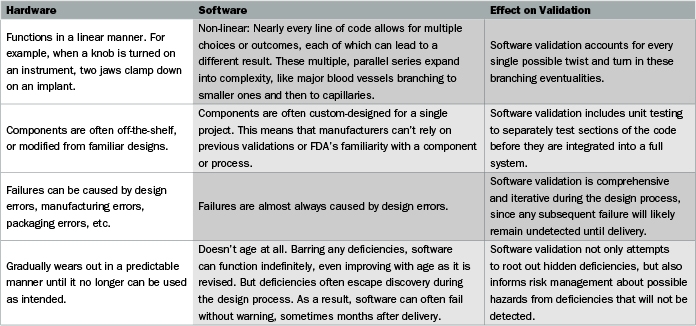
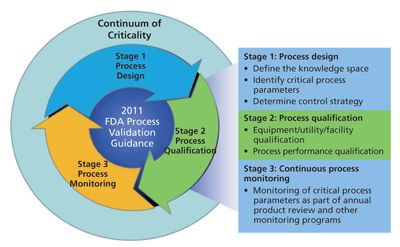
Ghtf study group 3 quality management systems process validation guidance january 2004 page 5 1 purpose and scope 1 1 purpose this process validation guidance is intended to assist manufacturers in understanding quality management system requirements concerning process validation.
A qms helps coordinate and direct an organization s activities to meet customer and regulatory requirements and improve its effectiveness and efficiency on a continuous.
This guidance serves as a.
Quality system information for certain premarket application reviews guidance for industry and fda staff pdf 548kb design controls.
This new guidance will provide guidelines for streamlining documentation with an emphasis on critical thinking risk management patient and product safety data integrity and quality assurance p.
The fda worldwide quality system requirements guidebook for medical devices.
Quality system regulation guidance documents.